Why Hepatitis A Should Be Included in India’s Universal Immunisation Programme
Introduction
As India debates inclusion of the Typhoid Conjugate Vaccine (TCV) into the Universal Immunisation Programme (UIP), experts argue that Hepatitis A vaccination deserves even higher priority.
A strong indigenous vaccine exists, disease patterns have changed, and the public-health risk is rising.
Changing Epidemiology of Hepatitis A in India
Traditional Pattern
Earlier: Most Indians infected in early childhood, usually with mild symptoms.
Result: Natural, lifelong immunity.
Current Pattern
Due to improved hygiene and sanitation:
Fewer children infected early → reduced natural immunity.
Increasing susceptibility in adolescents and adults, where disease is much more severe.
Evidence of Changing Pattern
Declining seroprevalence:
From >90% two decades ago
To <60% in many urban regions now
Multiple outbreaks in Kerala, Maharashtra, Uttar Pradesh, Delhi.
Hospitals reporting:
Acute liver failure (ALF)
Clusters of severe disease
Deaths in young adults
Why Hepatitis A Is Now a Public-Health Threat
No specific antiviral treatment exists.
Recovery relies on supportive care only.
Increasing outbreaks despite improved sanitation.
Affects all socio-economic groups, including urban middle class.
Significant morbidity in:
Adolescents
College students
Young adults
The Hepatitis A Vaccine: Characteristics and Strengths
Efficacy and Duration
Protection: >90–95%.
Duration: 15–20 years or lifelong.
Types available
Live-attenuated vaccines
Inactivated vaccines
Indigenous Success Story
Biological E’s Biovac-A (live-attenuated):
Developed in India
In use for over 20 years in private sector
Excellent safety and efficacy record
Advantages over Typhoid Vaccines
No issues of:
Waning immunity
Antibiotic resistance
Carrier state
Single dose provides long-term protection
Already manufactured domestically
Comparison: Hepatitis A vs Typhoid
Parameter | Typhoid | Hepatitis A |
Treatment | Antibiotics effective (AMR rising but manageable) | No specific treatment |
Severity | Declining mortality | Increasing severity in older age groups |
Immunity patterns | Widespread exposure; vaccine needed | Natural immunity declining rapidly |
Vaccine dosing | Multiple doses | Single-dose (live) |
Cost-effectiveness | Good | Higher |
Programmatic complexity | Moderate | Simple |
Indigenous vaccine | Yes | Yes (Biovac-A) |
By criteria of disease burden, cost-effectiveness, vaccine durability, and implementation ease, Hepatitis A ranks higher for immediate UIP inclusion.
Why Hepatitis A Is a “Low-Hanging Fruit”
Single-dose
Long-lasting immunity
Strong indigenous manufacturing
Clear evidence of rising disease burden
No treatment options for severe illness
Fits well into UIP infrastructure
Can be co-administered with DPT/MR boosters
Proposed Way Forward for India
1. Start with high-burden States
Kerala, Maharashtra, Uttar Pradesh, Delhi
States with repeated outbreaks or falling antibody prevalence
2. Use existing UIP infrastructure
Co-administration with boosters
No new logistics needed
3. Conduct periodic serosurveys
Monitor immunity levels
Guide nationwide scale-up
4. Consider phased expansion to national level
Similar to Rotavirus, Pneumococcal, and Hepatitis B rollouts
Why Inclusion Is Urgent Now
Outbreaks increasing in frequency and severity
Growing susceptible population
Urbanisation and mobility accelerating transmission
One severe episode can cause acute liver failure even in healthy youth
India already has a trusted, cost-effective, indigenous vaccine
India’s health landscape is shifting—Hepatitis A is no longer a mild childhood disease but an emerging cause of severe liver failure in adolescents and young adults. With a safe, effective, indigenous single-dose vaccine already available and strong evidence of rising susceptibility, the case for adding Hepatitis A to the Universal Immunisation Programme is both scientifically sound and programmatically feasible.
Universal Immunisation Programme (UIP)
Introduction
The Universal Immunisation Programme (UIP) is one of the world’s largest public health programmes, delivering free vaccines against multiple life-threatening diseases to all children and pregnant women in India. It is implemented by the Ministry of Health and Family Welfare (MoHFW) under the National Health Mission (NHM).
Launched in 1985, expanded in 1992 as part of the Child Survival and Safe Motherhood Programme, and strengthened through Mission Indradhanush (2014) and Intensified Mission Indradhanush (IMI 2.0, 3.0, 4.0).
Objectives of UIP
Reduce child mortality and morbidity from vaccine-preventable diseases.
Provide universal access to immunisation free of cost.
Achieve equitable coverage across socio-economic groups.
Maintain cold chain and vaccine logistics through the Universal Vaccine Logistics Management System (UVLMS).
Introduce new and evidence-backed vaccines into the national schedule.
Target Groups
All infants (0–12 months)
Children up to 16 years (for booster doses)
All pregnant women
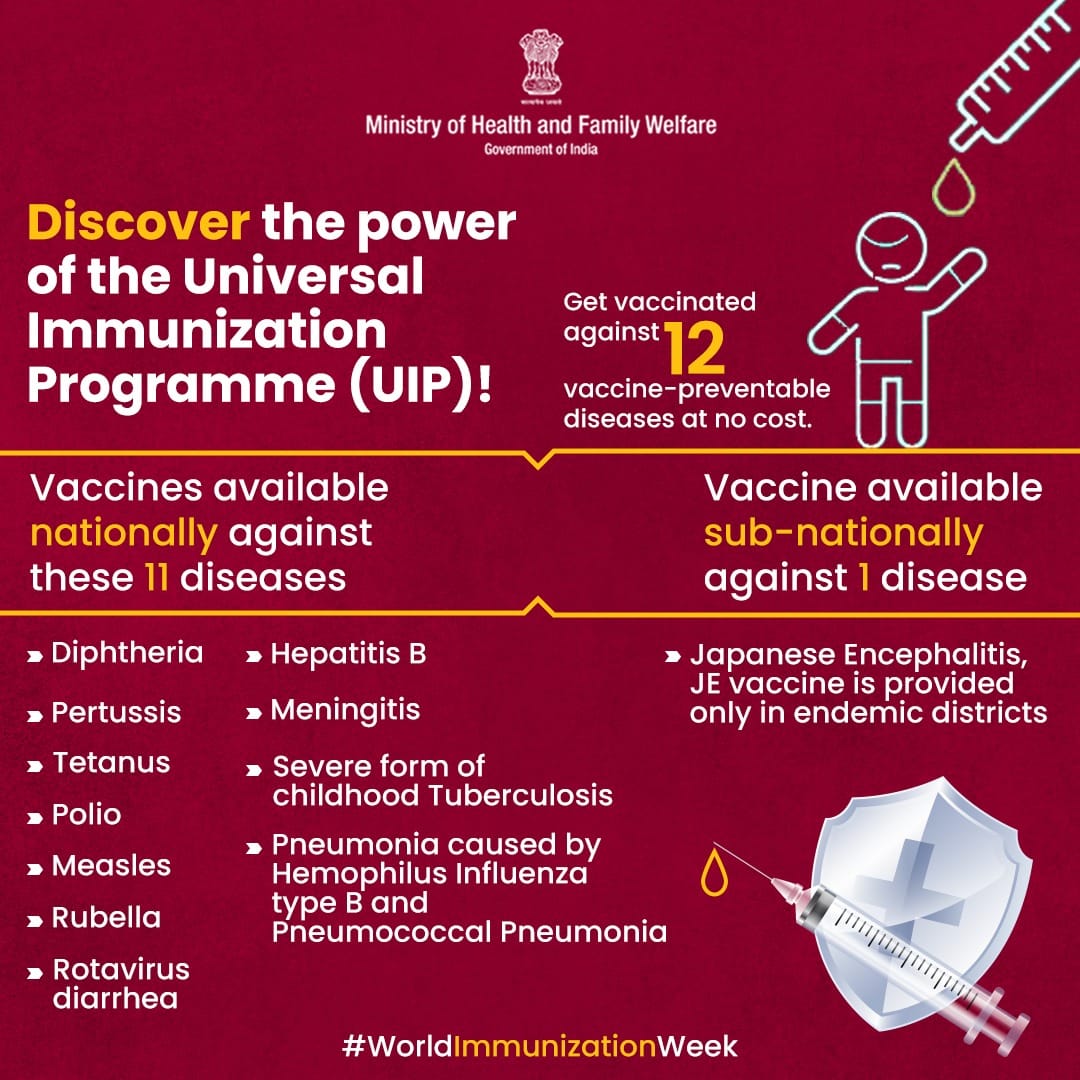
Diseases Covered Under UIP
The UIP currently protects against 12 life-threatening diseases:
Tuberculosis
Diphtheria
Pertussis (Whooping Cough)
Tetanus
Polio
Measles
Rubella
Hepatitis B
Haemophilus influenzae type b (Hib)
Rotavirus diarrhoea
Pneumonia and Meningitis (Pneumococcal disease)
Japanese Encephalitis (JE) – in endemic districts only
Vaccines Included Under UIP
1. BCG Vaccine
For Tuberculosis
Given at birth
2. OPV (Oral Polio Vaccine)
For Poliomyelitis
Multiple doses including birth dose
3. IPV (Inactivated Polio Vaccine)
Additional protection under polio endgame strategy
4. Hepatitis B Vaccine
For Hepatitis B virus (HBV)
Part of pentavalent vaccine
5. Pentavalent Vaccine (DPT + HepB + Hib)
Protects against Diphtheria, Pertussis, Tetanus, Hepatitis B, Hib pneumonia/meningitis
6. DPT Vaccine (Booster doses)
For Diphtheria, Pertussis, Tetanus
Given at 16–24 months and at 5–6 years
7. TT Vaccine (for pregnant women)
Replaced by Td (Tetanus-Diphtheria) since 2019
Prevents neonatal and maternal tetanus
8. Measles-Rubella (MR) Vaccine
Replaced Measles-only vaccine
Two doses: 9–12 months and 16–24 months
Critical for MR elimination targets
9. Rotavirus Vaccine
Prevents severe rotavirus diarrhoea
India uses indigenous vaccines (ROTAVAC & ROTASIIL)
10. Pneumococcal Conjugate Vaccine (PCV)
Prevents pneumonia and meningitis
Introduced nationally in 2021
11. Japanese Encephalitis Vaccine
Given in endemic districts (primarily in Uttar Pradesh, Assam, Bihar, West Bengal, etc.)
12. Vitamin A Supplementation (part of child health programme)
Prevents Vitamin A deficiency-related blindness and infections
Prelims Practice MCQs
Q. With reference to India’s Universal Immunisation Programme (UIP), consider the following statements:
It provides free vaccination to all infants, children up to 16 years, and pregnant women.
It is implemented under the National Health Mission (NHM).
It was first launched in 1992 as part of the Child Survival and Safe Motherhood Programme.
Which of the statements given above is/are correct?
A. 1 and 2 only
B. 2 and 3 only
C. 1 and 3 only
D. 1, 2 and 3
Answer: A
Explanation:
Statement 1 is correct.
Statement 2 is correct.
Statement 3 is incorrect: UIP was launched in 1985, not 1992.
Q. Which of the following diseases are currently covered under the Universal Immunisation Programme (UIP)?
Hepatitis B
Tuberculosis
Japanese Encephalitis
Rotavirus diarrhoea
Hepatitis A
Select the correct answer:
A. 1, 2 and 3 only
B. 1, 2, 3 and 4 only
C. 2, 3, 4 and 5 only
D. 1, 2, 4 and 5 only
Answer: B
Explanation:
UIP covers: Hepatitis B, TB, JE (in endemic areas), and Rotavirus among others.
Hepatitis A is NOT part of UIP yet, which is the central debate.
Q. With reference to vaccines used in the Universal Immunisation Programme (UIP), consider the following pairs:
Vaccine | Protects Against |
1. Pentavalent | Diphtheria, Pertussis, Tetanus, Hepatitis B, Hib |
2. PCV | Pneumonia and Meningitis |
3. MR | Measles and Rubella |
4. DPT Booster | Diphtheria, Pertussis, Tetanus |
Which of the pairs given above is/are correctly matched?
A. 1 and 2 only
B. 1, 3 and 4 only
C. 1, 2, 3 and 4
D. 2 and 4 only
Answer: C
Explanation:
All four pairs are correctly matched.
