Reducing Costs, Expanding Access: The Case for Simpler Biosimilar Testing
When a company develops a small molecule drug, it patents it, blocking competitors for years and allowing high pricing. Once the patent expires, competitors can produce generics, which are chemically identical, cheaper, and widely used in India—e.g., Sovaldi dropped from $84,000 to $1,000 with Indian generics.
Biologics, being complex and produced via biological systems, cannot be exactly replicated. Hence, generic versions are called biosimilars, not generics. Biosimilars require more rigorous, expensive testing than small molecule generics to prove efficacy and safety.
Globally, regulators like those in the U.K. and U.S. are reducing testing burdens by removing animal trials and adopting human-relevant methods. India still mandates animal studies but is considering easing norms, potentially aligning with global standards.
Biosimilars vs. Generics
| Biosimilars | Generics | |
|---|---|---|
| Type of Drug | Copies of biologic drugs (large, complex molecules made in living cells). | Copies of small molecule drugs (simple, chemically synthesized drugs). |
| Structure | Large, complex, and cannot be exactly replicated — only highly similar to the original. | Simple and identical in chemical structure to the original drug. |
| Production Process | Made using biological systems (living cells); sensitive to changes in process or environment. | Made by chemical synthesis; highly stable and reproducible. |
| Examples | Biosimilar insulin, biosimilar Herceptin (trastuzumab), biosimilar Remicade (infliximab). | Generic versions of paracetamol, amoxicillin, aspirin (Disprin), etc. |
| Cost | Cheaper than original biologics, but more expensive than generics due to complex production. | Much cheaper than branded drugs due to low production and development cost. |
| Approval Requirements | Requires comparative clinical studies, safety, efficacy, and immunogenicity data. | Requires proof of bioequivalence only (no need for full clinical trials). |
| Substitutability | Not always interchangeable; substitution policies vary by country. | Interchangeable with the original branded drug. |
| Regulatory Pathway | Specialized, more stringent pathway (e.g., WHO, US FDA, CDSCO biosimilar guidelines). | Simplified approval process under generic drug regulation. |
- Generics = exact chemical copies of small molecule drugs.
-
Biosimilars = close (not identical) copies of complex biologics, requiring more testing and regulation.
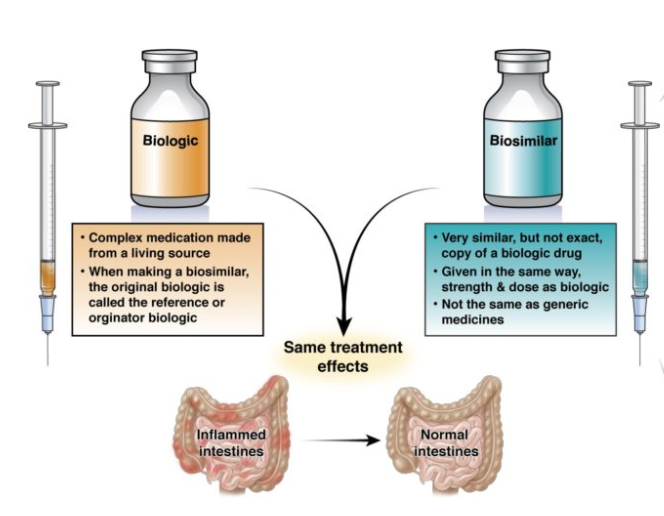
Biologics vs. Biosimilars
| Biologics | Biosimilars | |
|---|---|---|
| Definition | Complex medicines made from living organisms (e.g., proteins, antibodies). | Highly similar versions of approved biologic drugs, made by different firms. |
| Production | Produced using living cells through biotechnological processes. | Also produced using living systems but through different biological processes. |
| Structure | Large, complex, and not easily defined chemically. | Highly similar but not identical; slight variations exist. Biosimilars are not exact copies of the reference product due to the inherent variability in biological manufacturing processes. |
| Examples | Insulin, monoclonal antibodies (e.g., Remicade), vaccines. | Biosimilar versions of insulin, Herceptin, etc. |
| Cost | Very expensive due to R&D and manufacturing complexity. | Relatively cheaper than biologics but costlier than small-molecule generics. |
| Approval Process | Full clinical trials and safety tests required. | Requires evidence of similarity, but often needs additional tests (e.g., clinical trials, pharmacovigilance). |
| Regulatory Status | Approved as new drugs. | Approved based on similarity to an already approved biologic. |
| Substitutability | Original drug; standard treatment. | May not be automatically substituted; depends on national policy. |
-
Biologics are original, complex therapies derived from living organisms.
-
Biosimilars are near-copies of biologics, intended to be more affordable but still require significant testing to ensure safety and efficacy.
