India–UK FTA: A Double-Edged Sword for Access to Medicines
Context
-
The India–UK Free Trade Agreement (FTA) includes provisions on Intellectual Property (IP) that may impact the availability, affordability, and production of generic medicines.
-
Concerns are being raised by experts, academics, and access-to-medicine advocates.
Key Concerns in the FTA
A. Tilt Towards Patent Holders
-
IP clauses favour transnational pharmaceutical corporations.
-
Undermines public interest safeguards enshrined in the Indian Patents Act.
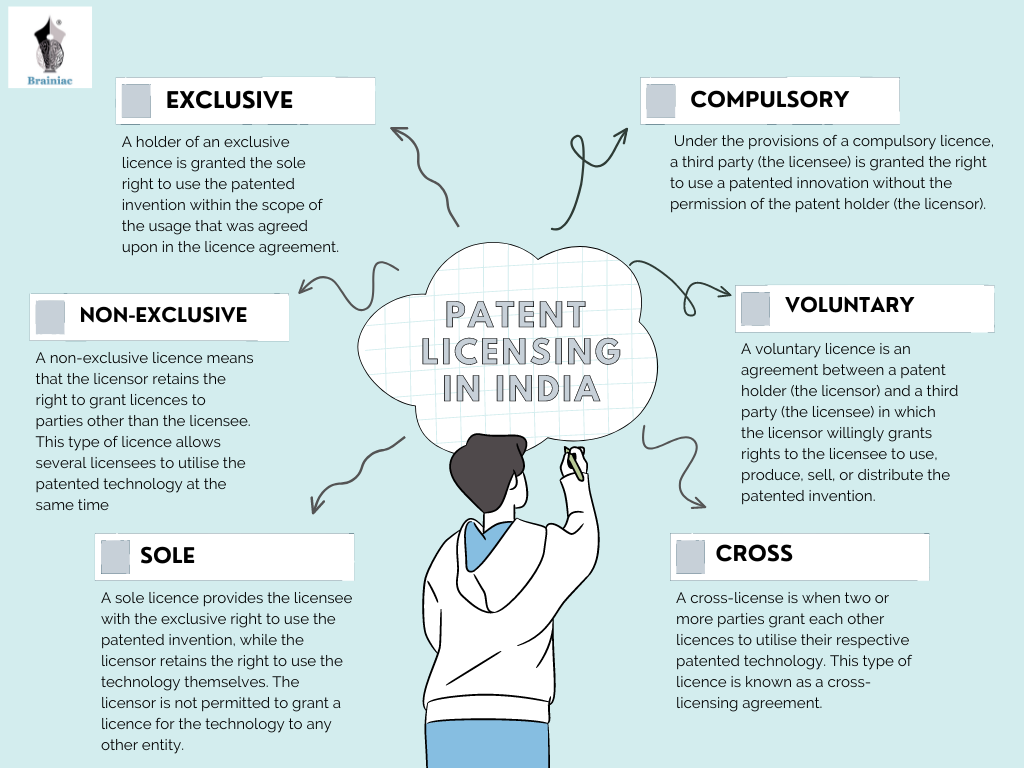
B. Voluntary Licensing vs Compulsory Licensing
-
Emphasis on voluntary licensing:
-
Relies on market forces, not state intervention.
-
Often comes with restrictive terms, royalty demands, and territorial limits.
-
Does not ensure substantial price reduction.
-
-
Reduces the government's power to issue Compulsory Licenses (CL), which were instrumental in past HIV/AIDS medicine access.
C. Dilution of Patent Working Requirements
-
Previously: Annual disclosure by patent holders on the working of the patent in India.
-
Now: Disclosure every 3 years.
-
Also, confidential information in reports will not be available publicly.
-
Implication: Harder to prove unmet needs—a ground for issuing CLs.
D. TRIPS-Plus Provisions
-
FTA includes TRIPS-Plus elements, such as:
-
Undermining Section 3(d) of Indian Patents Act (anti-evergreening clause).
-
Promoting harmonization of patentability standards.
-
Allows use of patent search/examination results from partner countries (may push for relaxed standards).
-
E. Evergreening Risk
-
Vague "best endeavour" clauses in IP chapter can dilute safeguards.
-
Could allow extension of patent life for minor modifications (e.g. new dosage forms) — blocking generics.
Supporters' Viewpoint
-
Trade proponents argue the FTA:
-
Gives zero-duty access for 99% of Indian pharma exports to UK.
-
UK pharma market set to grow from $45 bn (2024) to $73 bn (2033).
-
India's pharma exports to UK grew by 12.6% in FY24; generics alone at $910 million.
-
Reduced regulatory barriers = easier market entry + scope for India–UK healthcare collaboration.
-
Broader Implications
-
For India: Risks its global leadership in generic medicine production.
-
For Global South: Affordability of life-saving medicines in Africa, Latin America, Southeast Asia may be hit.
-
Contradicts India’s image as the ‘pharmacy of the developing world’.
Way Forward
-
Balance trade interests with public health safeguards.
-
Strengthen domestic pharma policies to:
-
Support MSMEs.
-
Ensure compulsory licensing remains robust.
-
-
Transparent monitoring of FTA implementation.
-
Protect Section 3(d) and public disclosure norms to ensure access to generics.
Voluntary Licensing
A voluntary license is a permission given by the patent holder (owner of the invention) to another party, allowing them to make, use, or sell the patented product or technology — with the patent holder’s consent and under agreed terms.
Key Features of Voluntary Licensing:
-
Mutual Agreement:
The patent holder voluntarily agrees to license their patent to another person or company — usually in exchange for royalties or a fee. -
Legal and Flexible:
It is a legal contract, and the terms (like duration, territory, royalty amount, etc.) are negotiated between the parties. -
Patent Owner Keeps Rights:
The original patent holder still owns the patent and can give licenses to multiple parties if they want. -
Used for Wider Access:
Voluntary licensing is often used in public health, such as allowing generic drug manufacturers to produce affordable versions of patented medicines, especially in developing countries.
Voluntary License vs. Compulsory License:
| Feature | Voluntary License | Compulsory License |
|---|---|---|
| Based on Consent? | Yes (by patent holder) | No (granted by government) |
| Usually with Royalties? | Yes | Yes, but often at lower rates |
| Common Use? | Pharma, tech, agriculture | Public health emergencies |
